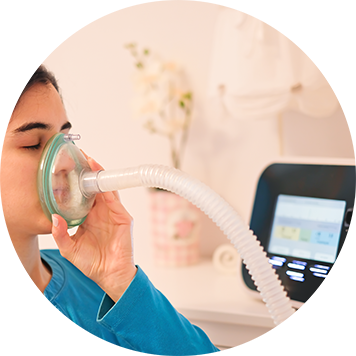
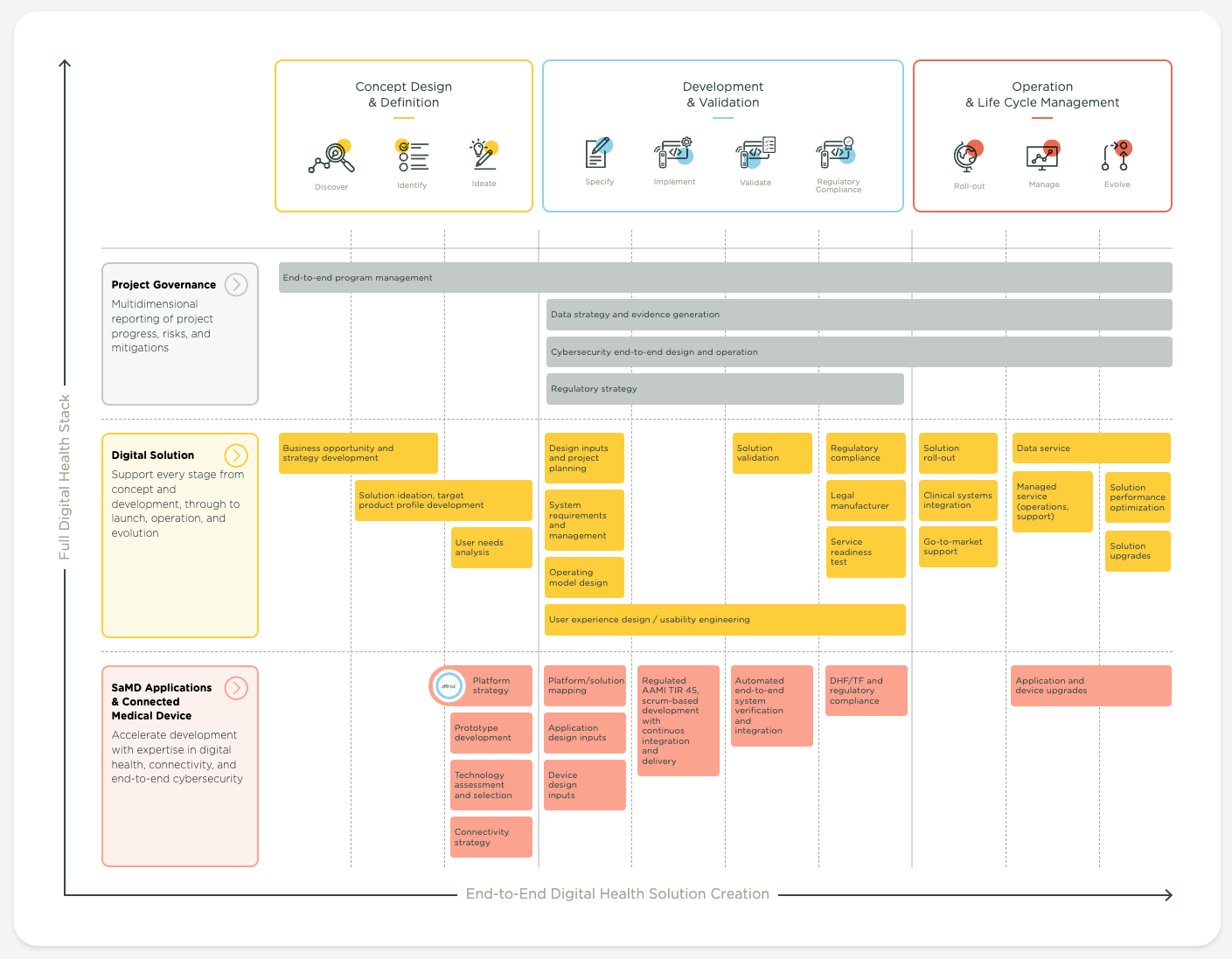
Support every stage from concept and development, through to launch, operation, and evolution
strategy development
product profile development
analysis
test
roll-out
Accelerate development with expertise in digital health, connectivity, and end-to-end cybersecurity
and selection
design
inputs
AAMI TIR 45,
scrum-based development
with
continuos integration
and
delivery
end-to-end system verification
and
integration
device upgrades